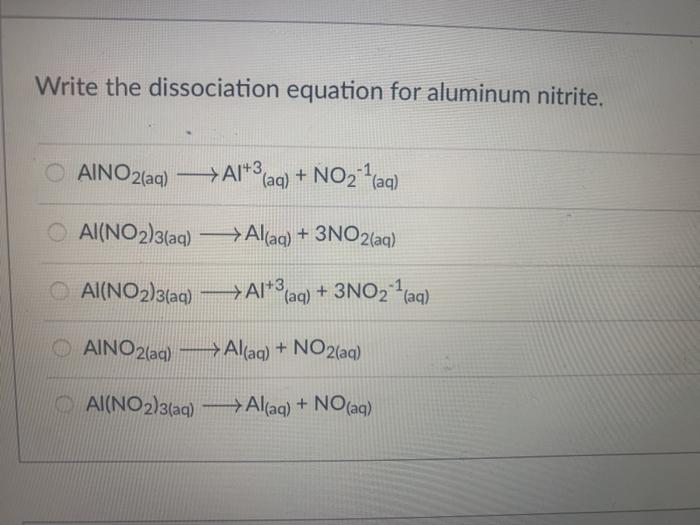
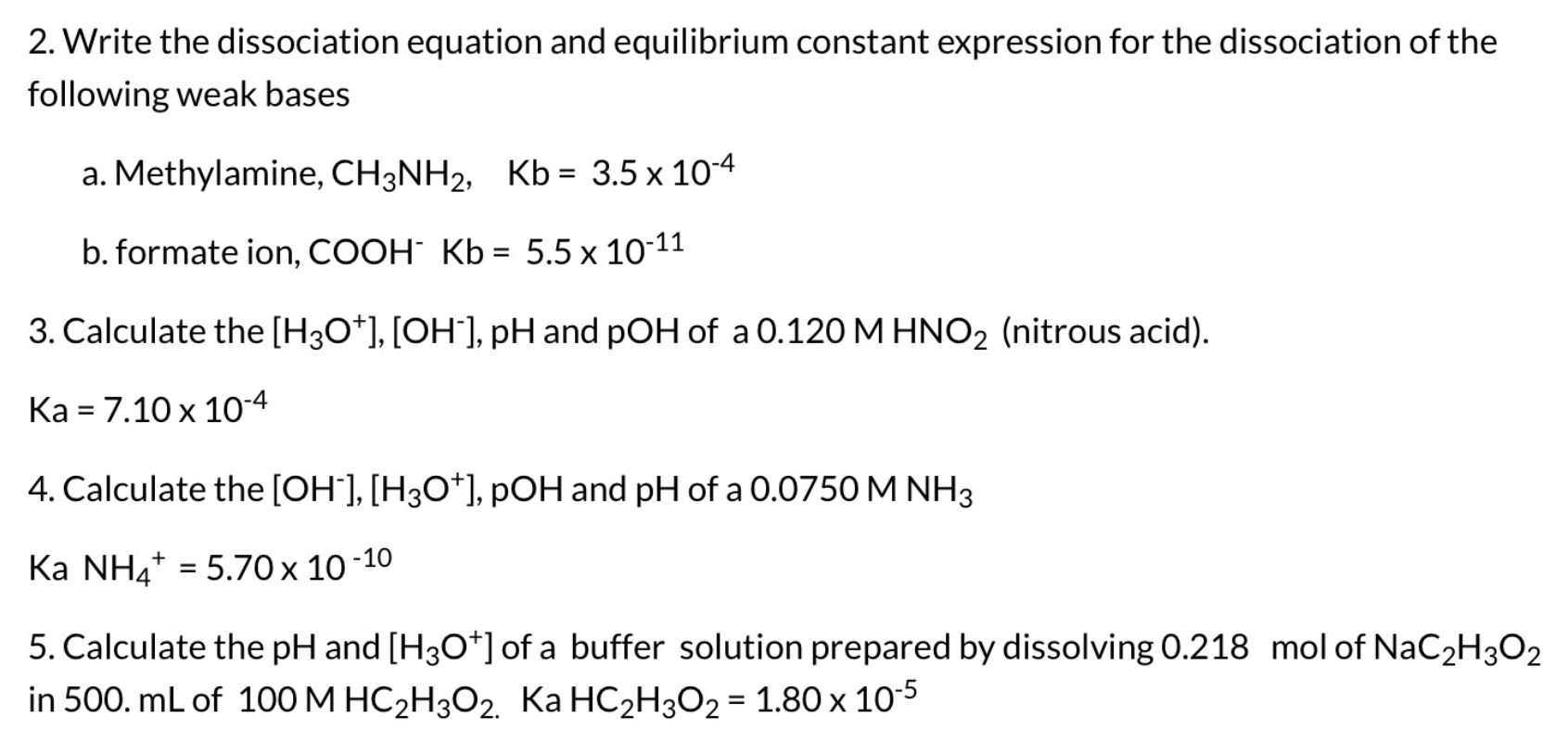
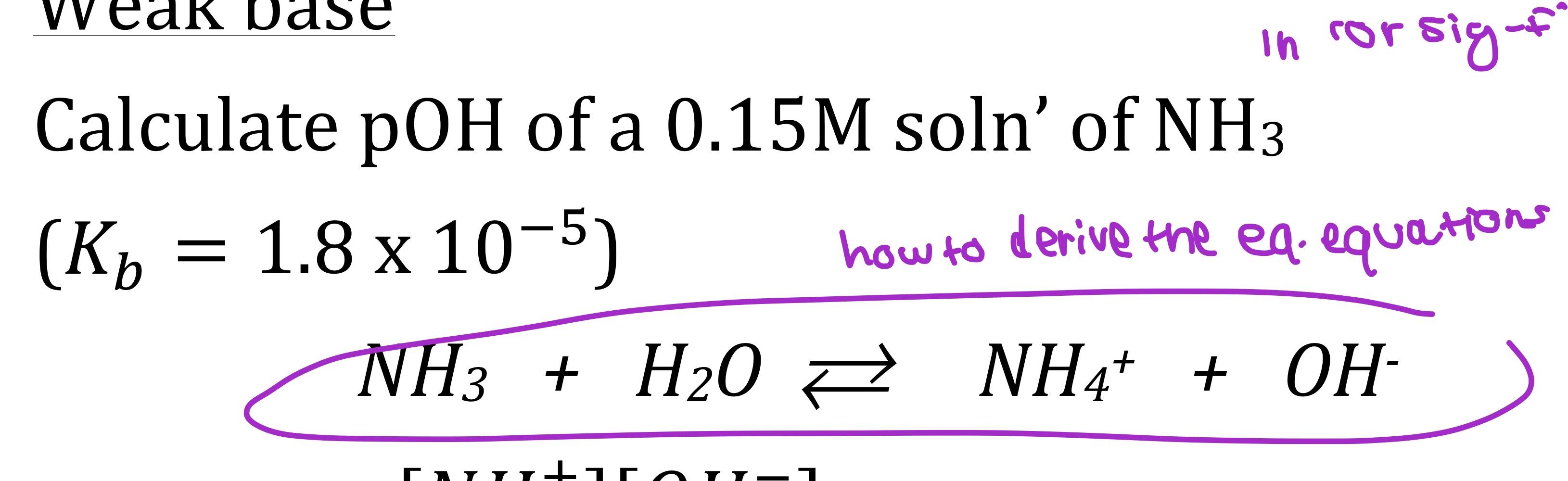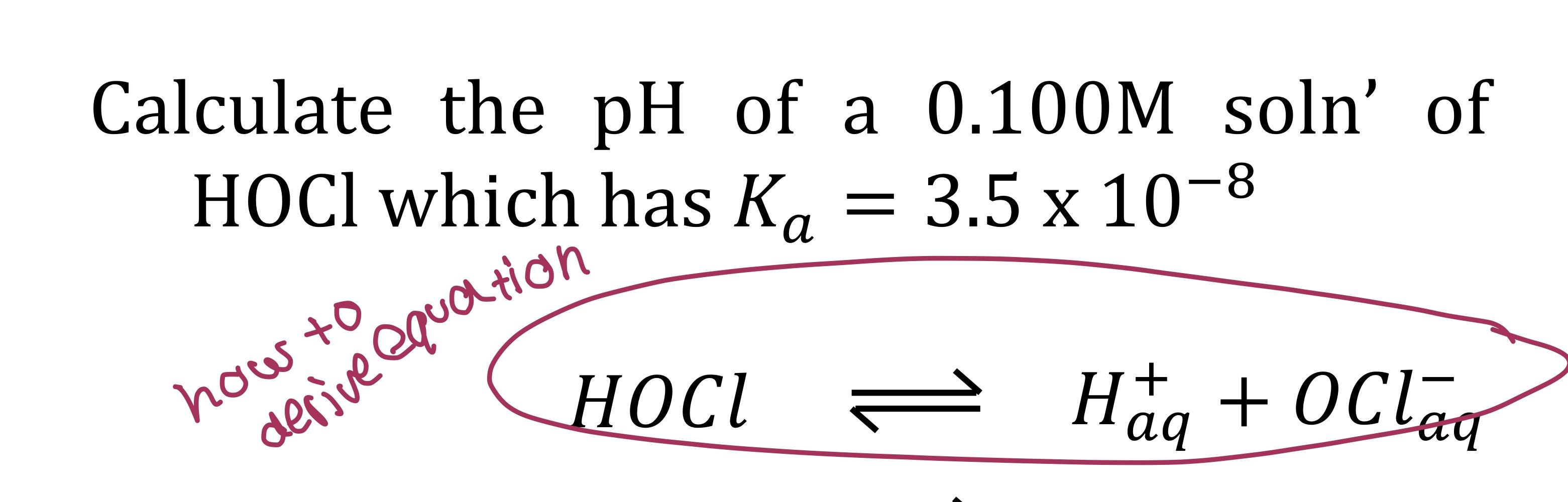Peerless Tips About How To Write Dissociation Equations
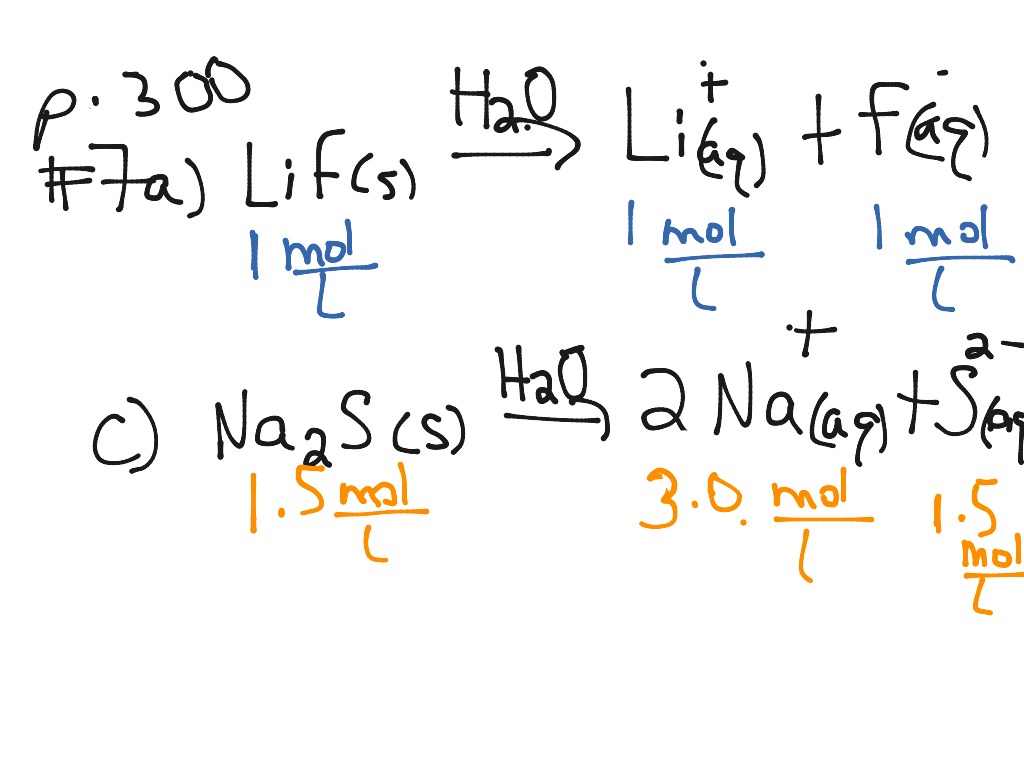
Simply undo the crisscross method that you learned when writing chemical formulas of ionic compounds.
How to write dissociation equations. Simply reverse the crisscross procedure you learned while writing ionic compound chemical formulas. Write the dissociation equations for the following strong electrolytes a) cocl3.more. The formula on the left consists of two sodium ions and one sulfide ion.
For example, the dissociation of. This chemistry video tutorial explains how to write dissociation equations of ionic compounds.introduction to. A dissociation reaction is a chemical reaction in which a compound breaks apart into two or more components.
In this video, i answer the following question: Your ionic charges are not correct for iodine. In this video, we will learn how to write equations for the dissociation constants of acids and bases and calculate their values.
It is important to be able to write dissociation equations. The dissociation equation must be balanced. When chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the.
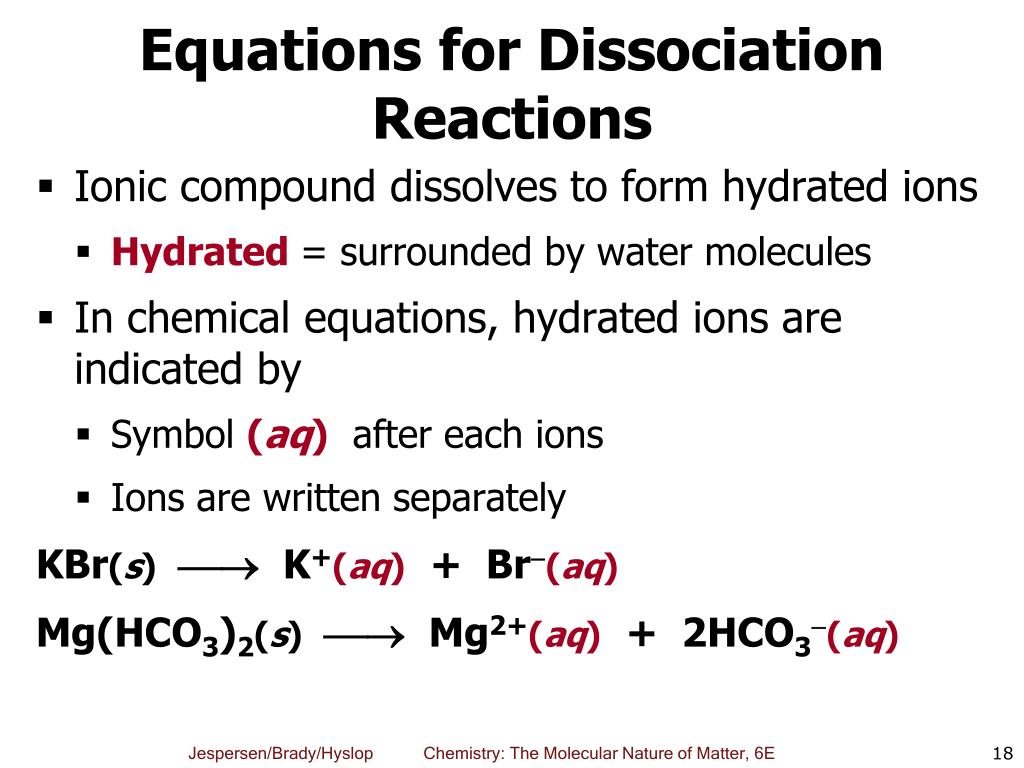
When chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the. Ab → a + b. For an aqueous solution of sodium hydroxide, we have.
Updated on october 21, 2019. The process for writing dissociation equations. You start with the a and b.
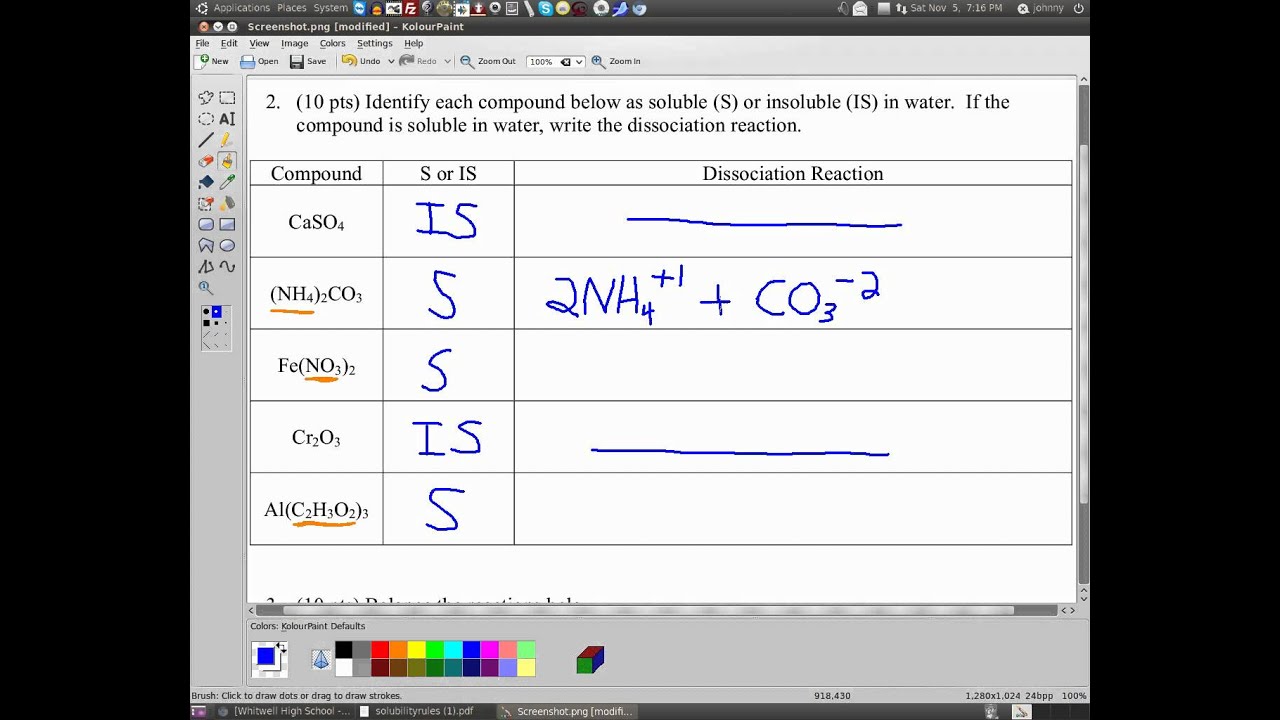
The equation for the dissociation of sodium sulfide in water is: The general formula for a. When we write a dissociation reaction to separate the two ions, place their charges above their symbols, and then balance the entire equation.
When the compound dissociates in. It is important to be able to write dissociation equations. 0:00 / 10:28.
Simply undo the crisscross method that you learned when writing chemical formulas of ionic compounds. Ab(aq) ⇌ a(aq) + b(aq) since dissociation reactions are typically reversible, the resulting atoms or. An easy way to remember this formula is to think of your dissociation as the breaking up of two components, a and b.
What is the process for writing dissociation equations of ionic compounds in mathematics education? It’s crucial to know how to write dissociation calculations. I would say we can write the following equations like this.

![[ANSWERED] 2) Write equations to show dissociation f... Physical](https://media.kunduz.com/media/sug-question/raw/82559353-1660075208.1950152.jpeg?h=512)

.PNG)